SATHGEN
State-of-the-art science for the hardest-to-treat cancers
At Sathgen Therapeutics, we are working on bringing first-in-class and best-in-class drugs ensuring novel treatments for difficult-to-treat cancers and viral infections.
Who We Are
Sathgen Therapeutics brings together strong leadership, world-renowned oncologists, and expert laboratory teams across India and the United States. With a robust pipeline and a compelling lead molecule, we are advancing innovative therapies for some of the hardest-to-treat cancers.





Our Trajectory
Our lead drug candidate has completed phase I clinical trials in patients with advanced solid tumors as well as in healthy individuals – to evaluate safety, tolerability and pharmacokinetics of the drug.
2023
Initiation of phase I trials of MSP008-22 in patients with advanced solid tumors.
2023
Initiation of phase I trials of MSP008-22 in healthy individuals.
2024
Completion of the dosing of the first cohort of patients in the phase I clinical trial of MSP008-22.
2025
Studies completed in healthy volunteers and patients with solid tumors.
Xenograft studies with triple negative breast cancer cells show tumor growth inhibition upon single arm MSP008-22 or in combination with standard-of-care chemotherapy, Carboplatin.
MSP008-22 improves the efficacy and lowers toxicity of another standard-of-care chemotherapy, Cisplatin, as shown by decreased IC50.
MSP008-22 shows excellent safety profile in pre-clinical models including induced human blood lymphocytes.
MSP008-22 also shows anti-stem cell and anti-metastatic efficacy in breast and prostate cancer models, in vitro.
Treatment with MSP008-22, both post-infection or prophylactic, reduces the SARS-CoV-2 viral load, and COVID-19-asscoiated Pneumonitis, alveolar injury, and inflammation score.
MSP008-22 inhibits viral replication to the same extent as the anti-viral, Remdesivir.
MSP008-22 shows anti-SARS-CoV-2 potential as observed in cytopathic assays, comparable to Remdesivir.
Financial and Technical Support
Sathgen Therapeutics has strong financial and technical support to help achieve its goals. Drug discovery and clinical trials are conducted at Sathgen division of Godavari Biorefineries Limited (GBL), and linked to the Somaiya Group, which is actively involved in healthcare and education.
Partner with us
We are actively advancing MSP008-22 toward clinical development and welcome strategic partnerships to accelerate its path to market.
Join our journey as we discover novel and effective treatments for hard to cure cancers.
Why Sathgen
Lead Compound
Demonstrated in vitro and in vivo efficacy against triple-negative breast cancer and prostate cancer.
Strong Backing
Opportunity to contribute to significant advancements in cancer treatment with potential for financial returns.
Robust Pipeline
Over 40 preclinical compounds targeting a diverse range of cancers. One compound in Phase 1
Impactful Investment
Opportunity to contribute to significant advancements in cancer treatment with potential for financial returns.
Drug Class and Indications
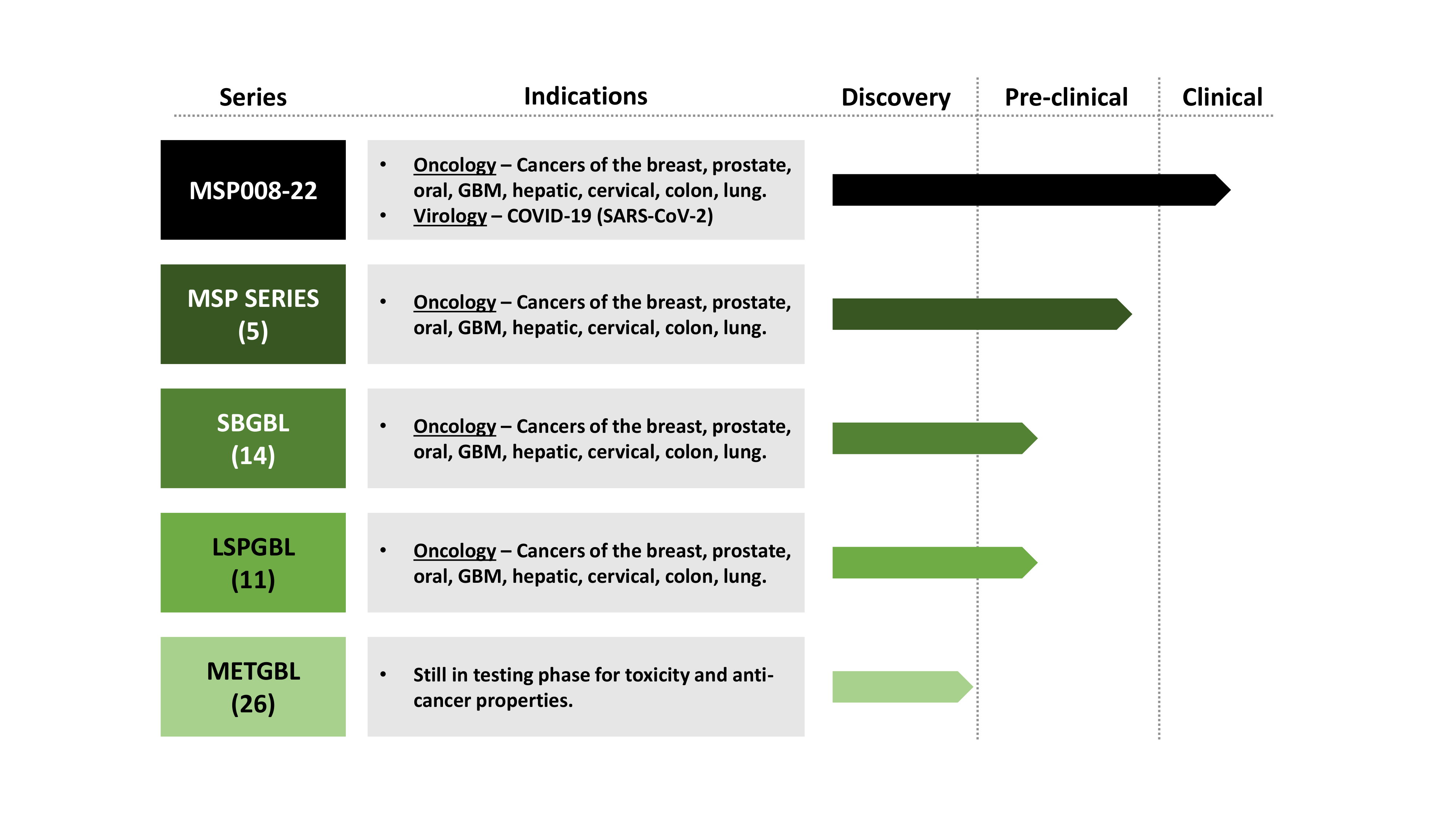
Global Patents
Drug Development & Commercialization
Patent for MSP008-22 anti-cancer compound granted in USA and Japan.
Patent for Synthesis of Cleistanthin A and its derivatives thereof granted in India, USA, Germany, Netherlands, France, UK, Mexico, South Africa, Australia, New Zealand, Israel, Japan, and Canada.
Patent for Process for Synthesis of Cleistanthin granted in India, Australia, New Zealand, Mexico, Canada, UK, France, Netherlands, Germany, and South Africa

Strategic Partnerships
Strategic partnerships are central to our growth. We invite pharmaceutical companies and research organizations to collaborate with us in advancing our therapeutic programs. Whether through licensing agreements, co-development initiatives, or other partnership models, we are eager to work with visionary partners who share our goal of transforming cancer care.
